Intervals of the duration time of different chemical reactions
per a unit of space vary from parts of a second to minutes, hours, days. Some reactions
are known to need several years, decades and even longer periods of time for their
continuance. If a reaction goes in a homogeneous system, then it is going in the entire
volume of this system. As a result of the reaction, as a rule, a heterogeneous system
appears:
H2SO4 + Na2S2O3
= Na2SO4 + H2O + SO2 + S
+ S
With any monophase mixture, the liquid solution of different substances can serve as
examples of a homogeneous system. If a reaction is going between substances, forming a
heterogeneous system, then it can go only on the surface of a phase division forming the
system. So, for example, a dissolution of a metal in an acid Fe + 2HCl = FeCl2
+ H2 can go
only on the surface of the metal because it is only here that both reacting substances
come into contact one with the other. The result of the reaction is again a heterogeneous
system, which under the conditions of lack of locking by means of a dismissal of one of
its phases can become a homogeneous system. As examples of heterogeneous systems we can
designate the following systems: some water with ice, a saturated solution with sediment,
sulphurs in the atmospheric air. At higher stages of the Evolution of Matter as examples
of homogeneous systems can be brakes of plants functionally of the same type (a forest,
meadow grass, orchards), united groups of animals functionally of the same type (a herd
of sheeps, a pack of wolves or monkeys). Heterogeneous systems in this case will be:
a herd of horses at a meadow, a team of lumbermen in a forest, production enterprises,
etc. Chemical kinetics is engaged in the study of conditions having an influence on
velocities of chemical reactions. At higher stages of the Evolution of Matter these
problems should be referred to the biological and to the social kinetics accordingly.
can go
only on the surface of the metal because it is only here that both reacting substances
come into contact one with the other. The result of the reaction is again a heterogeneous
system, which under the conditions of lack of locking by means of a dismissal of one of
its phases can become a homogeneous system. As examples of heterogeneous systems we can
designate the following systems: some water with ice, a saturated solution with sediment,
sulphurs in the atmospheric air. At higher stages of the Evolution of Matter as examples
of homogeneous systems can be brakes of plants functionally of the same type (a forest,
meadow grass, orchards), united groups of animals functionally of the same type (a herd
of sheeps, a pack of wolves or monkeys). Heterogeneous systems in this case will be:
a herd of horses at a meadow, a team of lumbermen in a forest, production enterprises,
etc. Chemical kinetics is engaged in the study of conditions having an influence on
velocities of chemical reactions. At higher stages of the Evolution of Matter these
problems should be referred to the biological and to the social kinetics accordingly.
The following factors are referred to as the most important,
having an influence on velocities of reactions, that go in systems of the level F:
functional peculiarities of reacting substances, their concentrations, temperature, the
presence of catalysts in a system. Velocities of some heterogeneous reactions depend also
on the intensity of the flow of a liquid or a gas near the surface, where a reaction is
going. After entering into a reaction of fng. units of two different substances fng. units
of a third, a fourth, and etc. substance is being created, which fill in fnl. cells
corresponding to them, though theoretically the process is occurring in the opposite
order: at first an invisible fnl. cell (C) of a new quality appears, then there
is the closing in of obvious fng. units (a and b) and the creation of a
new fng. unit (c), which fills in the fnl. cell (C), are going. Therefore
velocities of reactions depend on a capacity of reacting substances because of their
structural constructions to create new fng. units, that is of spatial locations and
mutual connections of initial fng. units of qualitative sublevels, on proportion and
quantity of fng. units (a and b) entering into reactions, that is
characterised by their concentrations.
Their mutual closing in and collision of one with another
(costroke) is the necessary condition so that between particles (molecules, ions) of
initial substances a chemical interaction would occur. Speaking precisely, particles
should approach each other so much, that atoms of one of them would feel the influence
of electrical fields originated by atoms of the other one. Only in such a case would
those transitions of electrons and regroupings of atoms become possible, resulting in
the formation of molecules of new substances - products of a reaction. However, not
every collision of molecules of reacting substances leads to the origination of the
product of a reaction. In order that a reaction occurs, that is new molecules form, it
is necessary to break or to weaken the connections between the atoms in molecules of
initial substances. That requires the spending of some energy. If colliding molecules
do not have enough energy, then their collision would not lead to the formation of
a molecule: having come into a collision they fly away in different directions like
elastic balls.
If the kinetic energy of colliding molecules is enough to weaken
or to break the connections, then a collision can initiate a reorganisation of atoms and
the formation of a molecule of a new substance. Therefore only those molecules that have
a surplus of energy in comparison with the average reserve of energy of all molecules can
overcome such an 'energetic barrier' in order to get into a chemical contact with each
other. The surplus energy that molecules should have in order that their collision could
lead to the formation of a new substance is named the energy of activation of a given
reaction. The molecules that have such energy are named active molecules. The surplus
energy of those molecules can be forward or rotary for a molecule as a whole, vibratory
for atoms, forming it, the energy of excitement for electrons, etc. For each specific
reaction only one kind of surplus energy can be principal. With a rise of temperature
the number of active molecules is increasing and as a result of that the velocities
of chemical reactions are accelerating as well.
The energy of activation of different reactions is different.
Its magnitude is the factor by which the influence of reacting substances tells on the
velocity of a reaction. For some reactions the energy of activation is insufficient,
for others, on the contrary, it is more than enough. If the energy of activation is too
insufficient, then it means that most collisions between particles of reacting substances
lead to a reaction. The velocity of such a reaction is high. On the contrary, if the
energy of activation is more than enough, then it means that only a very small number
of collisions of interacting particles leads to a chemical reaction. The velocity of
such a reaction is very little.
The reactions, which require some appreciable energy of
activation in order to move, start from the breaking or weakening of connections between
atoms in molecules of initial substances. During it the substances are getting over into
an unsteady intermediate state, which is characterised by a large reserve of energy -
an activated complex. Precisely for the formation of which the energy of activation is
essential. An unstable activated complex is in existence for a very short time. It is
decomposing with the formation of the products of the reaction, during which energy is
going out. In a simplest case an activated complex is a configuration of atoms, in which
the previous connections are weakened and new ones are being formed. An activated complex
arises as an intermediate state during both direct and reverse reaction. Energetically
it differs from initial substances by a magnitude of energy of activation of a direct
reaction and from final substances - by energy of activation of a reverse reaction.
Activation of molecules is possible during the heating or dissolution of a substance,
while emitting energy during a reaction itself, while absorbing by them quantums of
radiation (light, radio-active, X-ray, etc.), under an effect of supersound or of
electrical discharge and even from strokes into sides of a jar.
The velocity of a reaction often depends on the presence in
a system of the 'third' component, with which reagents can compose an activated complex.
During that an alteration of the velocity of a reaction occurs owing to the alteration
of the energy of its activation as intermediate stages of the process would be different.
The additional component, which is named a catalyst, after the destruction of the activated
complex, does not form part of the products of a reaction, therefore the general equation
of the process remains the same. In most cases the effect of a catalyst can be explained
by the fact that it reduces the energy of activation of a reaction. In the presence of a
catalyst the reaction is going through different intermediate stages, whereas without it,
moreover, those stages energetically are more accessible. In other words, in the presence
of a catalyst different activated complexes arise, while for their formation less energy
is required than during the formation of activated complexes that arise without a catalyst.
Thus the energy of activation is going down - some molecules, the energy of which was
insufficient for active collisions, now become active.
If a reaction A + B 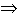 AB is going with a slow velocity, then it is
possible to find a substance K, that forms an activated complex with one of the
reagents, interacting in its turn with another reagent:
AB is going with a slow velocity, then it is
possible to find a substance K, that forms an activated complex with one of the
reagents, interacting in its turn with another reagent:
A + B 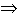 [A... K]; [A... K] + B
[A... K]; [A... K] + B 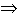 AB + K
AB + K
If the energy of activation of these stages is lower than
the energy of activation of the process in the absence of K, then the total velocity of
the process is increasing considerably and such a catalysis is named positive. Otherwise,
the velocity of the process would decrease and a catalysis would be negative. Thus a
catalyst is a substance that alters the velocity of a reaction and remains after that
chemically invariable. A catalyst, present in a system in quantities of a thousand times
less than reagents, can alter the velocity of a reaction by hundreds, thousands, millions
of times. In certain cases under the effect of catalysts such reactions can be excited,
which without them practically do not go on in the given conditions. At the same
time, with the help of a catalyst it is possible to alter the velocity only of a
thermodynamically possible process. For slowing down undesirable processes or for
giving reactions more quiet character negative catalysts are used.
One can discern a homogeneous and a heterogeneous catalysis.
In case of a homogeneous catalysis the catalyst and reacting substances form one phase
(a gas or a solution). In case of a heterogeneous catalysis the catalyst is in the system
in the form of an independent phase and the reaction takes place on its surface.
The catalysis plays a very important part in biological systems.
Ferments - plain and complex proteins with big molecular mass - are active catalysts of
biological effect. Most of the chemical reactions going on in the digestive system, in
blood and cells of animals and men, are catalytic reactions. So, a saliva has the ferment
ptyalin, which catalyses the transformation of starch into sugar. The ferment pepsin,
present in the stomach, catalyses the desintegration of proteins. Half of an available
quantity of urea under ordinary conditions at the temperature 25oC is
decomposed by water during 3200 years, but in the presence of the ferment urease the
time of its 'half-transformation' at the same temperature is only 10-4 sec.
In total more than 30 thousand different ferments are functioning in the organism of
a man; each of them serves as an effective catalyst of the corresponding reaction.
On studying heterogeneous reactions, it is not difficult to
notice that they are closely linked with the processes of displacement of fng. units
of substances, entering a reaction, and new substances. So, to keep the process of the
burning of pieces of coal constant it is necessary that dioxide of carbon, forming during
this reaction, would be moved away all the time from the surface of the coal and new
quantities of oxygen would approach it. Therefore during a heterogeneous reaction one
can single out at least three stages:
1) supply of reacting substances;
2) a chemical reaction itself;
3) taking aside the products of the reaction.
The velocity of a chemical reaction, which in its turn can be divided into substages,
is determined by the velocity of the slowest substage. A stage, determining the velocity
of going of the reaction as a whole, is named the limiting stage. In one case it can be
a supply or taking aside substances, in another - a chemical reaction itself.
All chemical reactions are divided into irreversible and
reversible. Irreversible reactions are going till the end - until the complete consumption
of one of the reacting substances. Reversible reactions are going not till the end: during
a reversible reaction no one reacting substance is consumed completely. Consequently an
irreversible reaction can go only in one direction, and a reversible one - both in one and
in the reverse directions as well. At the beginning of a reversible reaction during the
mixture of the initial substances the velocity of the one-direction reaction is high and
the velocity of the reverse one is equal to zero. While a reaction is going on the initial
substances are being used up and their concentrations are declining. As a result of that
the velocity of the one-direction reaction is decreasing. At the same time products of
the reaction are being composed and their concentration is increasing. Owing to this the
reverse reaction starts going while its velocity gradually grows. When the velocities
of the one-direction and the reverse reactions become identical, the chemical (dynamic)
balance begins.
By changing the conditions a system is under - concentration
of substances, pressure, temperature - it is possible to alter the velocities of the
one-direction and the reverse reactions. Then the balance in the system is being broken
and moved in the direction of that reaction, the velocity of which became higher. So,
during the increase of the concentration of reagents, the velocity of the one-direction
reaction naturally is growing and the balance is being moved towards the one-direction
reaction, towards more output of products. More output of products can be obtained also
by systematically getting them out of the sphere of the reaction, which leads to the
decreasing of their concentration in the system and to the deceleration of the reverse
reaction in comparison with the one-direction one. For chemical systems, which contain
gaseous substances, changes of pressure have the same influence on the shift of the
balance as the changes of the concentration of gases. During that the velocity of that
reaction is changing more, in which more molecules of gases are participating. The
changing of temperature has influence on the displacement of the chemical balance for
processes accompanied by thermal effects. If a one-direction reaction is exothermal,
then the reverse one is endothermal, and vice versa. For reversible reactions the energy
of activation of an endothermal process is more the energy of activation of an exothermal
process. In its turn, the more Eact. is, the more the velocity of a reaction
depends on temperature. So, an increase of temperature is moving the chemical balance
toward an endothermal reaction, as a result of which heat is taken up and the system
is cooling down.
On comparing the changes of conditions under which a chemical
system is staying with its responding reaction to an outer influence, revealing itself
in the moving of the chemical balance, it is not difficult to notice that this reaction
always turns out to be opposite to the change of a condition. So, if the concentration
of some substance, which is in balance with other reacting substances, is being reduced,
then the balance is moving toward the reaction, increasing the concentration of this
substance. While increasing the pressure then that process starts going faster, which
decreases it, and during the rise in temperature - the process, that causes cooling of
the system. These observations form the chemical content of the general principle of
behaviour of systems, staying under given conditions in a state of the dynamic balance:
if a system, staying in balance, undergoes an influence from without by alteration of
some condition, determining the state of balance, then the balance in it is moving toward
the process, which leads to the reduction of the effect of the influence. This rule of
counteraction is known under the name the principle of La Chattily, formulated by him
in 1884.
Thus, for the carrying through of each chemical reaction
strictly definite reagents are needed in quantities providing the required going of the
reaction under a given temperature and other conditions at a definite velocity, which can
be commensurate with temporal intervals. Moreover, every chemical reaction, going under
given conditions, has its own definite systemic construction, constituting a combination
of fnl. cells which at certain moments are being filled in and set free by fng. units
corresponding to them according to the typical for a given reaction algorithm, reflecting
the moments of entering the reaction by reagents - fng. units, their possible interchange,
while all this is correlated with strictly definite periods of time, fixed by an
independent counter of time.

Level G
All the simplest and complex molecular compounds of the
levels D, E and F are dispersed along the surface of the Earth,
and in accordance with their aggregate state form part of the land, oceans and
atmosphere of the Earth.
The Evolution of Matter along the sublevel G was
going by forming new molecular compounds, which obtained more and more new functions
in accordance with the motion of Matter in quality
( ).
).
The differentiation of fnl. cells and formation of new
fng. units of the present level were going in the process of the continual combining
of fnl. cells of previous sublevels, integrating and modifying their structures,
semi-decomposition of these original microsystems to the units of lower sublevels.
The whole process of the Evolution of Matter along the sublevel
G has been going for more than 5 billion years in the geospheres of the Earth -
spherical covers of different density and composition. For the most part they are
atmosphere, hydrosphere and lithosphere (the Earth's crust), which penetrate one into
another, are in close interaction, consisting in the exchange of substance and energy,
and represent the common system, being pierced by the Sun's radiation.
The outer geosphere is the atmosphere, which in its turn divides
into three sub covers: troposphere, stratosphere and ionosphere. Each of these subspheres
is characterised by sharply expressed physics peculiarities and bears strictly definite
functional loading. The boundaries between these covers are expressed not so sharply and
their altitudes are changing both with the time and latitude of a place. The upper boundary
of the troposphere is within the bounds from 8 to 18 km. The troposphere unites more than
79% of the mass of atmosphere. The stratosphere is extended till the altitude of about
80 km, constituting approximately 20% of the total mass of the atmosphere. Above the
stratosphere is located ionosphere, having less than 0.5% of the total mass of the
atmosphere.
The troposphere, where almost all water steam is concentrated,
is characterised by almost full transparency with regard to the short-wave sun radiation
passing through it, and by considerable absorption of the long-wave (thermal) radiation
of the Earth, caused mainly by water steam and clouds. Therefore the troposphere is
warming mainly from the earthy surface, as a result of which is the drop of temperature
with altitude. In its turn this leads to the vertical mixing of air, the condensation
of water steam, and the formation of clouds, rain and snow. The composition of the
troposphere includes (by volume) 78.08% of nitrogen; 20.95% of oxygen; 0.93% of argon
and about 0.03% of carbonic acid gas. 0.01% consists of hydrogen, neon, helium, krypton,
xenon, ammonia, peroxide of hydrogen, iodine and others.
The composition of dry air in the stratosphere differs by
a very important peculiarity - by increasing with altitude both the total concentration
and relative content of ozone (three-atom oxygen). Ozone is being formed in the
stratosphere as a result of the dissociation of molecules of oxygen under the influence
of ultra-violet radiation of the Sun and the subsequent joining of the turned out atom
of oxygen with another molecule of oxygen. Ozone is located in the atmosphere in the
form of a diffused layer, extended from the Earth's surface approximately 60 km. If all
the ozone of the atmosphere concentrated in the form of the layer under the overground
pressure, then the pellicle with thickness 2 - 3 mm could be seen. Despite so
insignificant a quantity the importance of the ozone in the atmosphere is exceptionally
great due to the extremely strong absorption by ozone of the radiation of both the Sun
and the Earth. So, owing to being absorbed by ozone, the ultra-violet radiation of the
Sun almost does not reach the troposphere at all.
The ionosphere, the outer sphere of the atmosphere, gets the
diverse radiation of the Sun and stars. Its structure consists mainly of atoms of oxygen
and other substances.
Between the atmosphere and the solid stone earth-crust there
is an interrupted water cover - the hydrosphere, covering nowadays 70.8% (361 mln. sq.
km) of the surface of the Earth. It constitutes the aggregate of oceans, seas and
continental water basins. The chemical composition of the hydrosphere is expressed
by the following figures: O - 85.82%, H - 10.72%, Cl - 1.9%, Na - 1.05%, Mg - 0.14%,
S - 0.088%, Ca - 0.04%, K - 0.038% , etc. The age of the hydrosphere is not less than
2 bln. years. In the hydrosphere Life on Earth was originated for the first time. The
evolution of organisms went on here during the whole pre-Cambrian period, and only at
the beginning of the Palaeozoic era did animal and vegetable organisms start to move
gradually to land. The main component of the hydrosphere is water - one of the
most widespread substances on the Earth. A lot of this water is in the gaseous state
in the form of steams in the atmosphere; during the whole year it is situated in the
form of huge masses of snow and ice on the tops of high mountains and in Arctic regions.
In the depths of the Earth there is also water, soaking soil and rocks. Water has rather
high coefficient of polyfunctionality and bears a large spectrum of functions to be
fulfilled. Being the first cradle of the origin of Life, water in each organism
constitutes habitat, in which chemical processes, which provide the vital activity
of organisms, take place; moreover it itself participates in a large number of
biochemical reactions. In the form of different solutions water carries out the
functions of displacement (transportation) of different fng. units from the place
of their synthesis to the place of their functioning in the structure of organism.
Being a highly reactionary capable substance, water is an active chemical reagent;
very often it carries out the functions of a catalyst. Having an anomalously high
thermal capacity it serves as a natural thermal accumulator.
The solid body of the Earth has three main geospheres: the
nucleus of the Earth, the intermediate cover and the earth-crust. The radius of the
nucleus is about 3500 km. The intermediate cover fills the space from the nucleus'
surface to the lower surface of the earth-crust and has the thickness of about 2900 km.
The earth-crust, or the lithosphere, is the upper solid cover of the Earth with thickness
15 - 70 km; from above it is limited by the atmosphere and the hydrosphere. The earth's
crust has a stratified structure, various in different places. The uppermost layer is
occupied by sedimentary cover (the stratisphere). It is interrupted, has the depth to
10 - 15 km and consists of sedimentary rocks, among which clays and argillaceous schist
predominate. Sands and sandstone, limestone and dolomites constitute its smaller part.
The formation of the stratisphere began in the ancient
pre-Cambrian period and lasts until now. The total age of the earth's crust is defined
as 3 - 3.5 bln. years, but the age of the most ancient, accessible for observation,
pre-Cambrian geological formations rather exceed 2 bln. years. The sedimentary cover
was formed as a result of the lengthy process of differentiation of the lithosphere's
substance under the influence of tectonic moves, the solar energy and vital activity
of organisms. This process was accompanied by a complex interchange of substances between
the granite and basaltic covers of the Earth, from one side, and the atmosphere and the
hydrosphere, from the other. The chemical composition of the stratisphere together with
the salt composition of the ocean is close to the average composition of the earth's
crust as a whole.
During the geological history of the Earth natural alterations
of the inner structure and consistency of the earth-crust, of the relief of its surface,
of the character of outer and inner geological processes were going on. So, for instance,
the rocks of the most ancient Archaen era everywhere are much metamorphosed
(recrystallised), pierced by intrusions of magma and crumpled into folds. Along the entire
surface of continents mountains arose repeatedly, which went to ruins later on. During
proterozoa and after that the continents, while going down, were partly flooded with sea
and, after getting up, again turned into dry land. Simultaneously powerful moves of the
earth-crust went on in different places, as a result of which numerous mountain ranges
were arising, later ruined. Contemporary inner geological processes reveal themselves:
1) in slow raising and lowering of the earth's
surface at the rate of several centimetres per year in mountainous areas, but
the usual rate amounts to some millimetres per year;
2) in abrupt moves of some parts
of the earth-crust - earthquakes;
3) in volcanic eruptions.
As a result of the above geological processes and also under
the permanent influence of the atmosphere (including the sun and cosmic radiation), the
hydrosphere and the biosphere during two bln. years the formation of the principal layer
of the lithosphere - the soil - was taking place.
Its formation went on from friable rocks, that is from the
fng. units of the sublevels D - F: clays, loam, sandy loam and sands,
constituting the products of the weathering of magmatic, metamorphosed or dense
sedimentary rocks, deposited at places of their origination or, more often, having
undergone transfers and redeposits (often repeated) by fluid water or wind. The soil
consists of the firm, liquid (the soil solution) and gaseous (the soil air) parts.
In the firm part the principal mass share is usually occupied by the mineral part,
represented by small (most of them are from 1 mm to tenth and hundredth parts of micron)
particles of different minerals. The composition of soil is formed by the following
chemical compounds (in decreasing order): SiO2, Al2O3,
Fe2O3, K2O, Na2O, MgO, CaO, CO2,
Cl, SO4 and by many others. But the most valuable component of the soil is
humus - the final result of the functional development of Matter along the organisational
level G. The composition of humus is formed by different high-molecular acids,
among which groups of gumming, ulmic and fulvo acids have the greatest importance. Chains
of aromatic nuclei of two- and three-member phenols form the basis of complex molecules
of gumming acids. Different functional groups are joined to them: carbocsilic,
methocsilic, spirituous and others.
All the numerous chemical compounds of the sublevel G,
including also humus substances, constitute complex systemic formations, enclosing into
its fnl. cells fng. units of all the foregoing sublevels from a to E. Each
of these particles, in the form of a certain way of organised structures of Matter, bears
at its organisational level different functional loads, that considerably differ from each
other. However, as it was already at the previous stages of the Evolution of Matter, each
stable systemic formation of the sublevel G at a certain moment becomes a fng. unit
of the following organisational level - H (the biosphere). And as soon as the actual
point of the invisible line of the tensor of the Evolution of Matter moved from the level
G to the level H, immediately the level G remained out of bounds
of the sphere of actual development of Matter and became, as also all the foregoing
organisational levels, a supplier of functional half-finished products - fng. units
of its sublevel - for the formation of functional systems of the level H.
The humus horizon of the soil serves as a natural accumulator
of these half-finished products, consisting mainly from its organic substance. Being the
very upper layer of the soil and coming into direct contact with the atmosphere and partly
with the hydrosphere, the humus horizon has relatively small thickness. It varies in
different grounds from several centimetres to one, sometimes to 1.5 m. In areas of deserts,
half-deserts, mountains, etc., the humus horizon is practically absent. But even at those
places where it is sizeable, the content of humus in the upper part of the humus horizon
ranges from tenth parts of a percent to 15 - 18%. Thus the formation, functioning and
development of fnl. systems and fng. units of all following organisational levels of
Matter depends directly on the quantitative composition of half-finished products being
situated in the humus horizon - the accumulator. But as this accumulator for many millions
of years has practically an invariable area (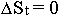 ), it serves as one of the principal natural
regulators of numbers of all living things on the Earth just in the same degree,
as all living things on the Earth themselves in order to avoid the worst
consequences should self-regulate its numbers in accordance with the resources
of this stage of the systemic organisation of Matter.
), it serves as one of the principal natural
regulators of numbers of all living things on the Earth just in the same degree,
as all living things on the Earth themselves in order to avoid the worst
consequences should self-regulate its numbers in accordance with the resources
of this stage of the systemic organisation of Matter.
![]() + S
+ S![]()
![]() can go
only on the surface of the metal because it is only here that both reacting substances
come into contact one with the other. The result of the reaction is again a heterogeneous
system, which under the conditions of lack of locking by means of a dismissal of one of
its phases can become a homogeneous system. As examples of heterogeneous systems we can
designate the following systems: some water with ice, a saturated solution with sediment,
sulphurs in the atmospheric air. At higher stages of the Evolution of Matter as examples
of homogeneous systems can be brakes of plants functionally of the same type (a forest,
meadow grass, orchards), united groups of animals functionally of the same type (a herd
of sheeps, a pack of wolves or monkeys). Heterogeneous systems in this case will be:
a herd of horses at a meadow, a team of lumbermen in a forest, production enterprises,
etc. Chemical kinetics is engaged in the study of conditions having an influence on
velocities of chemical reactions. At higher stages of the Evolution of Matter these
problems should be referred to the biological and to the social kinetics accordingly.
can go
only on the surface of the metal because it is only here that both reacting substances
come into contact one with the other. The result of the reaction is again a heterogeneous
system, which under the conditions of lack of locking by means of a dismissal of one of
its phases can become a homogeneous system. As examples of heterogeneous systems we can
designate the following systems: some water with ice, a saturated solution with sediment,
sulphurs in the atmospheric air. At higher stages of the Evolution of Matter as examples
of homogeneous systems can be brakes of plants functionally of the same type (a forest,
meadow grass, orchards), united groups of animals functionally of the same type (a herd
of sheeps, a pack of wolves or monkeys). Heterogeneous systems in this case will be:
a herd of horses at a meadow, a team of lumbermen in a forest, production enterprises,
etc. Chemical kinetics is engaged in the study of conditions having an influence on
velocities of chemical reactions. At higher stages of the Evolution of Matter these
problems should be referred to the biological and to the social kinetics accordingly.![]() [A... K]; [A... K] + B
[A... K]; [A... K] + B ![]() AB + K
AB + K![]()